The RURAL Cohort Study team has 16 member institutions made up of researchers across the nation.
These institutions include professionals in a variety of fields, all working to promote the health of your community. Each institution works as an important component, providing research answers, and community engagement for participants and community members.
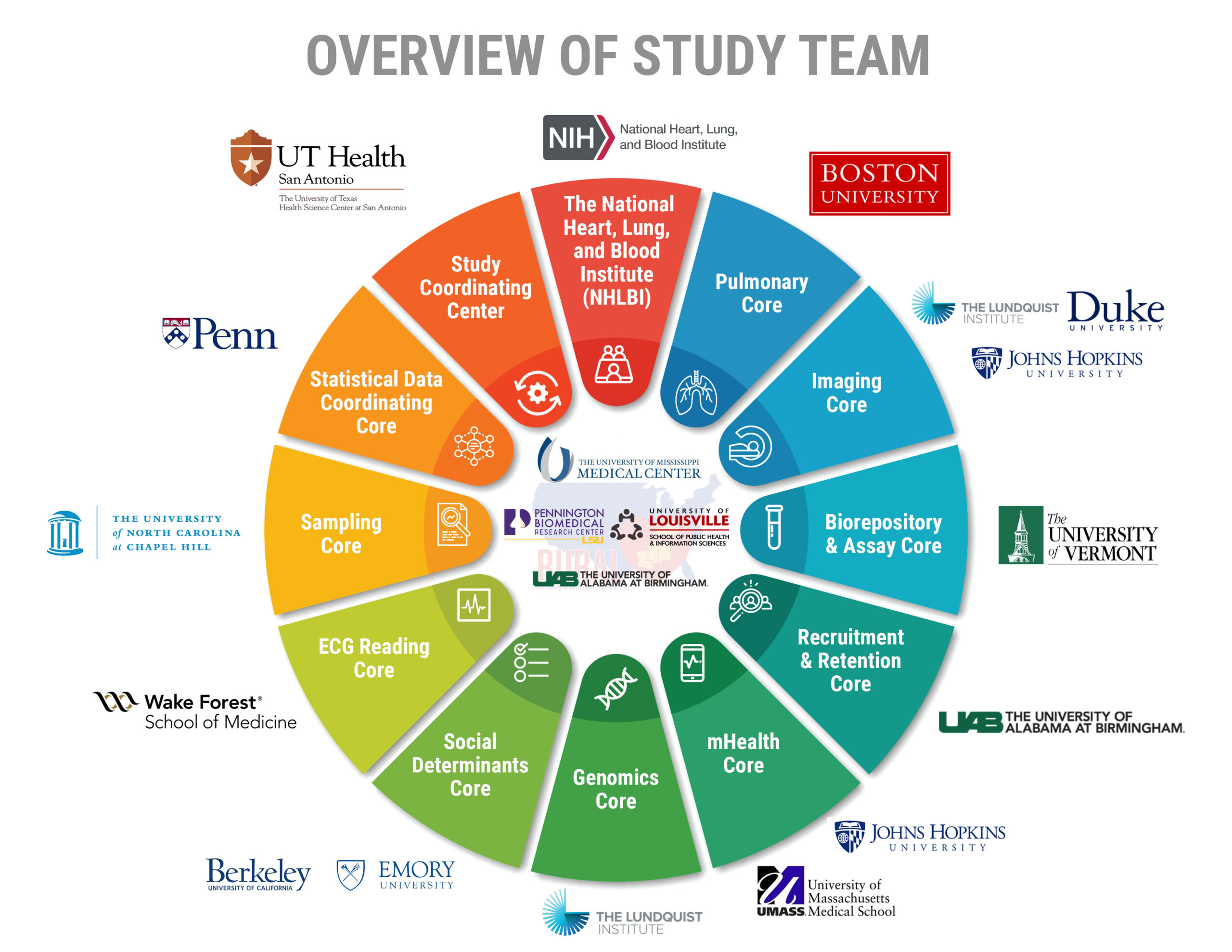

The RURAL Cohort Study is funded by The National Heart, Lung, and Blood Institute (NHLBI), which provides global leadership in research, training, and education to prevent and treat heart, lung, blood, and sleep disorders.
The NHLBI has appointed an Observational Study Monitoring Board (OSMB), which is common for epidemiological studies involving multiple sites (such as The RURAL Study). The principal role of the OSMB is to regularly monitor the data generated in the study, and assess the performance of study operations. The OSMB will make recommendations regarding issues related to participant burden, safety, confidentiality, and informed consent, including the plans for returning results to participants. The OSMB will review the RURAL Cohort Study’s progress in terms of recruitment, quality control, data analysis, publications, impact of proposed ancillary studies, and overall achievement of the main study goals.
The Study Coordinating Center (SCC) of the RURAL Cohort Study is based out of The University of Texas Health Science Center at San Antonio under the leadership of Contact Principal Investigator, Vasan Ramachandran, MD. The SCC will be responsible for overseeing the overall management of the study, including investigators, cores, and RURAL infrastructure. The SCC will also coordinate with the NHLBI project office and the various external monitoring boards and committees.


The Pulmonary Core of the RURAL Cohort Study is based out of Boston University under the leadership of Hector Marquez, MD along with his colleague, George O’Connor, MD. The Pulmonary Core is responsible for the measurements of minor and major spirometry.
The Alabama State and Recruitment and Retention Core (RRC) of the RURAL Cohort Study is based out of the University of Alabama at Birmingham (UAB) under the leadership of Drs. Suzanne Judd and Shauntice Allen, as well as David Rhodes, RN. UAB is responsible for leading the RURAL Cohort Study efforts within the state of Alabama, including recruiting and retaining participants in two counties. Additionally, the RRC will be responsible for the overall strategy of examining participants and managing MEU technicians across the 4 states. The RRC will also lead participant follow-up surveillance and endpoint adjudication. Finally, the RRC will handle clinically actionable medical alerts, where UAB has expertise from its role in the REGARDS study.


The Mississippi Core of the RURAL Cohort Study is based out of the University of Mississippi Medical Center under the leadership of Ervin Fox, MD, MPH, along with his colleagues, Frances Henderson, RN, EdD; Felicia Caples, PhD; Sonja Fuqua, PhD, RN; and Abril Grant. The core will be responsible for leading the community engagement efforts within the state of Mississippi, including assisting in the recruitment of participants in two counties in Mississippi.
The Louisiana Core of the RURAL Cohort Study is based out of the Pennington Biomedical Research Center (PBRC) at Louisiana State University under the leadership of Stephanie Broyles, PhD, along with her colleagues, Corby Martin, PhD, and Denise Holston, PhD of the LSU AgCenter. The core is responsible for leading the community engagement efforts within the state of Louisiana, including assisting in the recruitment of participants in two parishes in Louisiana.


The Kentucky Core of the RURAL Cohort Study is based out of the University of Louisville under the leadership of Stephanie Boone, PhD, MPH, along with her colleagues, Kathy Baumgartner, PhD, and Richard Baumgartner, PhD. The core is responsible for leading the community engagement efforts within the state of Kentucky, including assisting in the recruitment of participants in four counties in Kentucky.
The Imaging Core represents a collaboration between three institutes: the Lundquist Institute, Duke University, and Johns Hopkins University. The core is led by Matthew Budoff, MD and Gerald Bloomfield, MD, along with their colleague, Michael Blaha, MD, MPH. The Imaging Core is responsible for obtaining CT scans; storing and processing of medical images; and the management, transmission, storage, and access of images.


The Biorepository and Assay Core (BRAC) of the RURAL Cohort Study is based out of the University of Vermont under the leadership of J. Peter Durda, PhD, along with his colleagues, Russell Tracy, PhD, Elaine Cornell, and Rebekah Boyle. The BRAC Core is responsible for collecting and processing biological samples, measuring selected key metabolic and cardiovascular biomarkers, and storing samples for future research use.
The mHealth Core represents a collaboration between the University of Massachusetts Medical School and Johns Hopkins University. The core is led by David McManus, MD, and Michael Blaha, MD, MPH. The mHealth Core is responsible for mobile technology development and deployment, as well as monitoring participants’ use of the wearable activity monitors.


The Genomics Core for the RURAL Cohort study is based out of the Lundquist Institute under the leadership of Jerome Rotter, MD, along with his colleagues, Yii-Der Ida Chen, PhD, Xiuqing Gua, PhD, Henry Lin, MD, and Kent Taylor, PhD. The Genomics Core is responsible for production of large-scale genotype data, quality control, and statistical genetic analyses on RURAL participants.
The Social Determinants Core represents a multi-institutional collaboration between the University of California, Berkeley and Emory University. The core is led by Mahasin Mujahid, PhD, along with her colleagues Viola Vaccarino, MD, MPH, Gene Brody, PhD, Ana Diez Roux, MD, PhD, MPH, Joel Kaufman, MD, MPH, and Tené Lewis, PhD. The Social Determinants Core is responsible for assessing multi-level contextual, psychological, social, and environmental determinants of health among participants.


The ECG Reading Core for the RURAL Cohort Study is based out of Wake Forest University under the leadership of Elsayed Z. Soliman, MD, MS. The ECG Reading Core is responsible for collecting digital ECG tracings using mobile electrocardiographs in the MEU, transmitting digital ECG tracings over a secure network, and storing data for research use.
The Sampling Core of the RURAL Cohort Study is based out of the University of North Carolina at Chapel Hill under the leadership of Robert Agans, PhD, along with his colleague, William Kalsbeek, PhD, MPH. The Sampling Core is responsible for designing the sampling strategy for the RURAL Cohort Study and ensuring the enrollment of participants across ten counties.


The Statistical and Data Coordinating Core (SDCC) for the RURAL Cohort Study is based out of the University of Pennsylvania under the leadership of Jesse Y. Hsu, PhD, MS, along with his colleague, Qi Long, PhD. The SDCC is responsible for data collection, processing, and analysis for multidisciplinary research projects across the cores, working groups, and repositories. In addition, they provide operational support and training to each RURAL Cohort Study site for implementing protocols, quality assurance, and control. The SDCC also provides biostatistical support and methodological innovation.
RURAL Cohort Study News
UVM Researchers Tackle Rural Health Disparities
April 2024
Members of the Biorepository and Assay Core (BRAC) at the University of Vermont discussed their involvement in the RURAL Cohort Study and how it was designed to uncover risk and protective factors for rural populations in the South.
The RURAL Study Participated in NHANES Webinar
March 2024
The RURAL Study participated in a webinar hosted by the National Health and Nutrition Examination Survey (NHANES) in October 2023. The webinar included other state and local-level survey programs to discuss experiences in multimode health and nutrition data collection. The webinar addresses topics s …